Introduction
Myelofibrosis (MF) is a myeloproliferative neoplasm (MPN) which can be primary or secondary to Essential Thrombocytopenia (ET) or Polycythemia Vera (PV). Despite all the therapeutic advances in this field over the last decade, allogeneic stem cell transplant (aHSCT) is the only available curative modality. According to literature, the main cause of mortality in the MF patients undergoing aHSCT is relapse (41-61%) and there is currently no standardized strategy to prevent relapse. Studies showed that the presence of GVHD was associated with a lower incidence of relapse. A quicker tapering of immunosuppressive drugs (IS) has been shown to increase the rate of GVHD and in some studies improve patients' outcome in adverse AML. At our center, starting in January 2017 we applied for all patients transplanted for MF a quicker tapering of IS, with all IS being weaned at 3 months instead of 6 months. We report here the outcome of MF patients transplanted with a reduced exposure to IS.
Methods
This is a retrospective, single center study. All patients transplanted for myelofibrosis between 2010 and 2022 at the university hospital of Nice were included. The indications for allogeneic stem cell transplant were based on the consensus of the EBMT/ELN international working group. We calculated the DIPSS plus score, MTSS score and the MIPSS70 plus score for all patients. In our institution, patients treated before 2017 had a longer immunosuppressive therapy with a duration of IS treatment of 6 months. Since 2017, the immunosuppressants were reduced from Day 60 post allo-HSCT to be weaned at day 100. The statistical study was carried out with SPSS software, Overall and relapse free survival were estimated according to Kaplan Meier and compared according to log rank.
Results
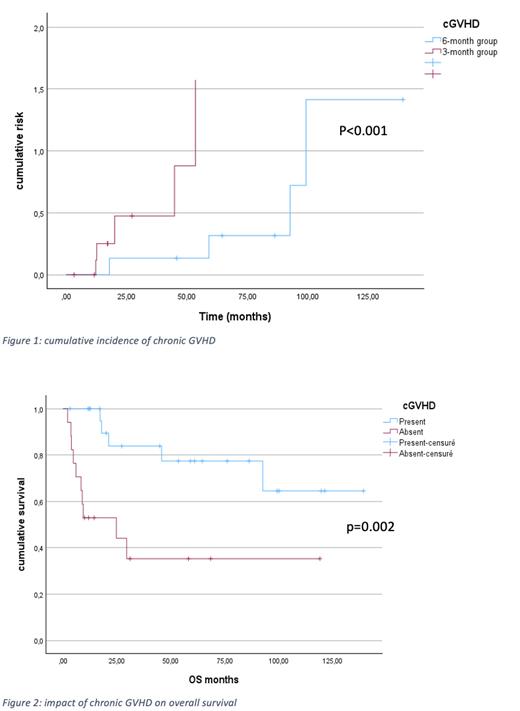
Forty-one patients have been transplanted and were included. Median age was 60.3 years (42-70). Thirteen are female and 28 are male. Twenty patients (48.8%) had primary MF according to the WHO classification and 21(51.2%) had a transformed MF from another MPN of which: 15 secondary to essential thrombocytopenia, 4 to polycythemia vera and 2 to a MPN not otherwise specified. Twenty-one patients were treated in the 6-month group and 20 patients in the 3-month group. There was no significant difference in terms of cumulative incidence of acute GVHD (p=0.902). Cumulative incidence of cGVHD was significantly higher in the 3-month group (P<0.001) (figure 1). Death has occurred in 15 cases (36.6%) with 10 cases (47.6%) in the 6-month group and 5 cases (25%) in the 3-month group. NRM was not significatively different between the two groups (0.998). 5-year GRFS 41.4% in the 6-month group and 54.1% in the 3-month group (p=0.513). Median follow up was 83.5 months. 5-year OS was 59.5% and 5-year RFS was 60.3%. The presence of chronic GVHD significantly improved OS in our study with a 2-y and a 5-y OS of 83.9% and 77.4% versus 52.9% and 35.3%(p=0.002) (Figure 2). The only score that predicted survival was the MTSS score (P=0.012).
Conclusion
In this series we found that a shorter course of immunosuppressive treatments resulted in a significantly higher incidence of cGVHD but not of aGVHD. In the entire cohort cGVHD was significantly associated with a better OS. The smaller size of our cohort makes it harder to identify an impact of the 3 months tapering strategy on OS.
A larger study is necessary to confirm the impact of a quicker tapering of IS on patients' survival.
Disclosures
Cluzeau:Incyte: Speakers Bureau; Servier: Consultancy, Speakers Bureau; Keros: Speakers Bureau; Syros: Speakers Bureau; Jazz Pharma: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau. Loschi:Pfizer: Honoraria; Jazz: Honoraria; Kartos: Honoraria; Medac: Honoraria; MSD: Honoraria; Novartis: Honoraria; AstraZeneca: Honoraria; Sanofi: Honoraria; Sobi: Honoraria; Telios: Honoraria; BMS: Honoraria; Gilead: Honoraria; GSK: Honoraria; Alexion: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal